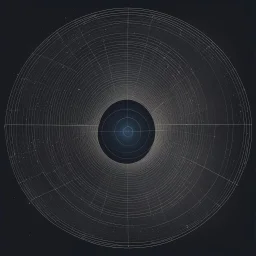
![Placeholder: [art by Zdzisław Beksiński] artistic and yet geometric representations of Bohr orbits: compares the electron probability densities for the hydrogen 1s, 2s, and 3s orbitals. Note that all three are spherically symmetrical. For the 2s and 3s orbitals, however (and for all other s orbitals as well), the electron probability density does not fall off smoothly with increasing r. Instead, a series of minima and maxima are observed in the radial probability plots](https://img.stablecog.com/insecure/64w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vYjBkODI0OGItNDk0MC00MjdlLTgxMjctMTViZjBlZGQ3N2FjLmpwZWc.webp)
![[art by Zdzisław Beksiński] artistic and yet geometric representations of Bohr orbits: compares the electron probability densities for the hydrogen 1s, 2s, and 3s orbitals. Note that all three are spherically symmetrical. For the 2s and 3s orbitals, however (and for all other s orbitals as well), the electron probability density does not fall off smoothly with increasing r. Instead, a series of minima and maxima are observed in the radial probability plots](https://img.stablecog.com/insecure/1920w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vYjBkODI0OGItNDk0MC00MjdlLTgxMjctMTViZjBlZGQ3N2FjLmpwZWc.webp)
@generalpha
Prompt
[art by Zdzisław Beksiński] artistic and yet geometric representations of Bohr orbits: compares the electron probability densities for the hydrogen 1s, 2s, and 3s orbitals. Note that all three are spherically symmetrical. For the 2s and 3s orbitals, however (and for all other s orbitals as well), the electron probability density does not fall off smoothly with increasing r. Instead, a series of minima and maxima are observed in the radial probability plots
11 months ago
Model
SSD-1B
Guidance Scale
7
Dimensions
1024 × 1024
![[art by kupka] artistic and yet geometric representations of Bohr orbits: compares the electron probability densities for the hydrogen 1s, 2s, and 3s orbitals. Note that all three are spherically symmetrical. For the 2s and 3s orbitals, however (and for all other s orbitals as well), the electron probability density does not fall off smoothly with increasing r. Instead, a series of minima and maxima are observed in the radial probability plots](https://img.stablecog.com/insecure/256w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vZDY2ZTVjN2UtMTJiNy00ZTBlLTk4ZjQtNTQ3MjhjOWVlMjQwLmpwZWc.webp)

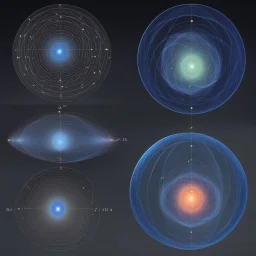
![[art by Paul Ranson] artistic and yet geometric representations of Bohr orbits: compares the electron probability densities for the hydrogen 1s, 2s, and 3s orbitals. Note that all three are spherically symmetrical. For the 2s and 3s orbitals, however (and for all other s orbitals as well), the electron probability density does not fall off smoothly with increasing r. Instead, a series of minima and maxima are observed in the radial probability plots](https://img.stablecog.com/insecure/256w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vMDgxNDc4MDAtMzFhOS00YTZjLTg3YmEtMzI0YjY2MGRkYWQ2LmpwZWc.webp)
![[abstract art inspired by Hieronymus Bosch and František Kupka] Out of the shadows emerged a group of machines, their metallic frames glinting ominously in the dim light. Their red eyes pierced through the darkness, scanning their surroundings with ruthless precision. Maria Magdalena stood her ground, her eyes locked with those of the machines, unyielding in her resolve.The storm raged outside, mirroring the turmoil within. Lightning illuminated the once bustling streets, briefly revealing the e](https://img.stablecog.com/insecure/256w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vYmZmYTY1OWYtMGVlNy00YjQ3LTg2MGYtMmUyNTA5YWY0MjgwLmpwZWc.webp)
![Quaternion and Hypercomplex Fractals Description: Generalization of Mandelbrot and Julia fractals using quaternion (4D) or hypercomplex numbers, then projected into 3D. Characteristics: More exotic and less intuitive shapes, often with “holes” or ribbon-like structures. [in fractales IFS with Apophysis refined in Mandelbulb 3D]](https://img.stablecog.com/insecure/256w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vZmRhN2M3OTgtYTg1YS00OTBlLTkyYjEtM2ExYTQ3YWZmZjJjLmpwZWc.webp)
![[art by Jean-Paul Riopelle] artistic and yet geometric representations of Bohr orbits: compares the electron probability densities for the hydrogen 1s, 2s, and 3s orbitals. Note that all three are spherically symmetrical. For the 2s and 3s orbitals, however (and for all other s orbitals as well), the electron probability density does not fall off smoothly with increasing r. Instead, a series of minima and maxima are observed in the radial probability plots](https://img.stablecog.com/insecure/256w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vMDViNjQzZDItODUxYy00MzNmLTg4ZjktZTUzY2RlZjExMTg4LmpwZWc.webp)
![[kupka] Destination: Void (Frank Herbert)](https://img.stablecog.com/insecure/256w/aHR0cHM6Ly9iLnN0YWJsZWNvZy5jb20vZjhlNzE1YWEtZGRiOS00NDY4LTk5YzEtMDZmMzVlNWM1MDMxLmpwZWc.webp)

